Okuqukethwe
Ukuhlaziywa kweBicarbonate
Incazelo yama-bicarbonates
The ion bicarbonates (HC03-) bakhona egazini: badlala indima enkulu kwi umthethonqubo we-pH. Ziyizona “okuyisidididi” esikhulu zomzimba.
Ngakho-ke, ukugxila kwabo egazini kulingana ngqo ne-pH. Ngokuyinhloko izinso ezilawula ukuhlushwa kwama-bicarbonate egazi, okukhuthaza ukugcinwa kwawo noma ukuphuma kwawo.
Ukulawula i-pH, i-bicarbonate ion HCO3- ihlangana ne-H ion+ ukunikeza amanzi ne-CO2. Ingcindezi ku-CO2 egazini legazi (Pa CO2), noma i-capnia, noma ingcindezi eyingxenye eyenziwe yi-CO2 encibilikiswe egazini lokuhlangana, ngakho-ke nakho kuyinkomba yokulinganisa kwe-acid-base. Kukalwa ngesikhathi sokuhlaziywa kwamagesi egazi.
Ama-bicarbonate ions ayisisekelo: lapho ukugxila kwawo kwanda, i-pH nayo iyanda. Ngakolunye uhlangothi, lapho ukuhlushwa kwabo kwehla, i-pH iba yi-acidic.
Kumuntu onempilo, i-pH yegazi izinze kakhulu: 7,40 ± 0,02. Akufanele yehle ngaphansi kuka-6,6 noma inyukele ngaphezu kuka-7,7, okungahambisani nempilo.
Kungani ukuhlaziywa kwe-bicarbonate?
Umthamo we-bicarbonate ions wenza kube nokwenzeka ukuhlola ibhalansi ye-acid-base yegazi. Kwenziwa ngesikhathi esifanayo nokuhlaziywa kwamagesi egazi, lapho udokotela esola ubukhona bokungalingani kwe-acid-base (i-acidosis noma i-alkalosis). Lokhu kungaba njalo lapho kukhona izimpawu ezithile, njenge:
- isimo esishintshile sokwazi
- i-hypotension, okukhipha inhliziyo okuphansi
- iziyaluyalu zokuphefumula (ukukhohlisa noma ukuphefumula kanzima).
- Noma ezimeni ezingathi shu kangako njengokugaya ukudla okungajwayelekile noma ukulahleka komchamo noma ukuphazamiseka kwe-electrolyte.
Ukubuyekezwa kwama-bicarbonates
Ukuhlolwa kwegazi kuqukethe isampula yegazi elinemithambo yegazi, imvamisa lisongweni lendololwana. Akukho ukulungiselela okudingekayo.
Yimiphi imiphumela esingayilindela ekuhlaziyweni kwama-bicarbonates?
Ukuhlaziywa kwenza kube nokwenzeka ukuxilonga ubukhona be- i-acidosis noma i-alkalosis. Isilinganiso se-pH sizokuvumela ukuthi ubone ukuthi ngabe kukhona yini i-hyperacidemia (echazwa njengenani le-pH elingaphansi kuka-7,35) noma i-hyperalcalemia (inani le-pH ngenhla kuka-7,45).
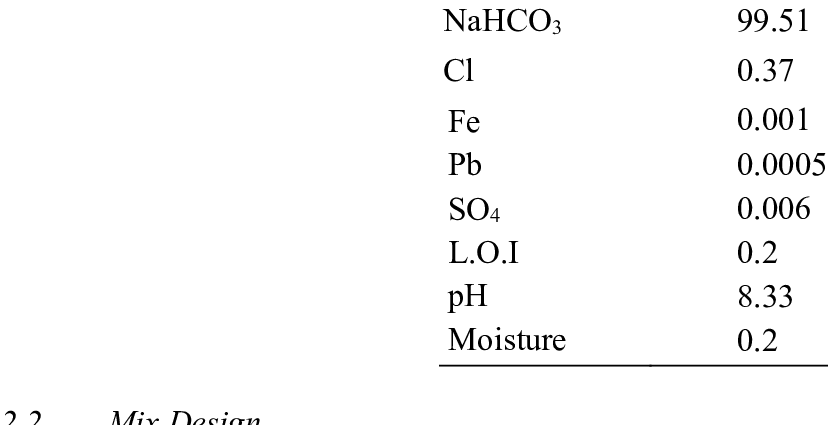
Ukukalwa kwama-bicarbonate ions ne-PaCO2 bese ivumela ukunquma ukuthi ngabe ukuphazamiseka kungokwemvelo ye-metabolic (ukungajwayelekile kwama-bicarbonates) noma ukuphefumula (ukungajwayelekile kwe-PaCO2). Amanani ajwayelekile ama-bicarbonate aphakathi kuka-22 no-27 mmol / l (ama-millimoles ilitha ngalinye).
Ukwehla kokuhlungwa kwama-bicarbonate ion ngaphansi kwamanani ajwayelekile kuba nomphumela i-metabolic acidosis. I-Acidosis ixhunyaniswa nokweqile kwama-H + ions. Uma kwenzeka i-metabolic acidosis, kuzoba nokwehla kokugcwala kwama-ion e-bicarbonate (pH <7,35). Ku-acidosis yokuphefumula, ukukhuphuka kwengcindezi eyingxenye ye-CO2 okuzoba nesibopho sokwanda kwama-H + ions.
I-metabolic acidosis ingabangelwa, phakathi kwezinye izinto, ukulahleka okungavamile kwama-bicarbonates ngenxa yohudo noma ukumuncwa kasawoti womzimba.
Ngakolunye uhlangothi, ukwanda kokuhlushwa kwama-carbonate ions kuholela ku i-alkalosis yomzimba (pH> 7,45). Kungenzeka uma kwenzeka ukuphathwa ngokweqile kwama-bicarbonates, ukuhlanza okukhulu noma ukulahleka kwe-potassium (isisu, isifo sohudo, ukuhlanza). I-Hyperaldosteronism nayo ingabandakanyeka (i-hypersecretion ye-aldosterone).
I-alkalosis yokuphefumula, ngokwengxenye yayo, ihambelana nokwehla okukodwa kwengcindezi engaphelele ye-CO2.
Funda futhi: Konke mayelana ne-hypotension |